Introduction
In HR+/HER2- metastatic breast cancer (MBC), concurrent aromatase inhibitor (AI) and CDK4/6 inhibitor is standard first line therapy. Mutations in the gene ESR1, which are found upon disease progression in approximately 40% of patients, confer treatment resistance to AI.1 When this occurs, patients are switched from AI to a selective estrogen receptor degrader (SERD) such as elacestrant (oral) or fulvestrant (intramuscular) while CDK4/6i is continued. In clinical practice preceding SERENA-6, ESR1 mutation is found after tissue biopsy once disease progression has occurred. Notably, the recent phase III PADA-1 trial found that ctDNA monitoring of ESR1 mutation to inform an earlier treatment switch showed a clear clinical benefit (HR = 0.61, 95% CI: 0.43 – 0.86).2
The purpose of the SERENA-6 study was to determine whether the emergence of an ESR1 mutation can be reliably monitored by ctDNA liquid biopsy and whether a survival advantage is gained using that information to drive the decision to switch treatment to a novel oral SERD, camizestrant. The rationale for the SERENA-6 study was that if the emergence of an ESR1 mutation can be recognized earlier, switching treatments before clinical progression might improve outcomes.
SERENA-6 was a phase III double-blind, randomized, multi-center controlled trial of adults with HR+/HER2- MBC who had received at least 6 months of AI + CDK4/6i, had no radiologic evidence of progression, and agreed to surveillance of ESR1 mutation every 2-3 months via liquid biopsy. A key feature of the intervention was 1:1 randomization triggered by detection of ESR1 mutation through Guardant360 CDx. The intervention group received camizestrant + CDK4/6i + placebo (in place of AI) and the control group received AI + CDK4/6i + placebo (in place of camizestrant/SERD). The I.V. was treatment group while the D.V.s were progression free survival (PFS), second PFS (PFS2), overall survival (OS), objective response rate (ORR) as determined by investigators using RECIST 1.1 criteria, patient-reported health status, quality of life (QoL) as measured by the European Organization for Research and Treatment of Cancer (EORTC) questionnaire, and adverse events. At interim analysis, there was a statistically significant improvement in PFS in the intervention group vs the control group (16.0 vs 9.2-month median). Median time to deterioration in patient-reported health status and QoL was 23.0 months in the intervention group vs 6.4 months in the control group.
Use of Theory
The theoretical foundation for SERENA-6 hinges on our understanding of tumor biology, the mechanism of action of the drug and genetic test being investigated, and the results of prior clinical trials. Early detection of ESR1 mutation is believed to prevent clonal evolution, limiting tumor heterogeneity and improving PFS. This was demonstrated experimentally in PADA-1.2 Camizestrant antagonizes estrogen receptor signaling more effectively than fulvestrant, as reported in the SERENA-2 trial.3 Together, these trials were cited to argue in support of the SERENA-6 trial design, which compared early switching to camizestrant vs maintaining AI rather than including fulvestrant (which would have eliminated fidelity of blinding due to its route of administration) or elacestrant (approved based on data from the EMERALD trial published in May 2022, but preliminary data presented December 2021) as an oral SERD alternative to camizestrant.4
The implicit theory drove the development of the intervention. The intervention directly measures the emergence of the ESR1 mutation in ctDNA and the investigational drug antagonizes estrogen receptor function. Outcomes were theoretically relevant, as patients were expected to take longer to experience clinical progression and decreased QoL.
The concepts and their definitions are consistent with the implicit theory. The study design leveraged longitudinal blood sampling which captured the key concept of treatment resistance. I think that the investigators’ decision-making is logical, however I think better care could have been taken to select the treatment administered to the control group. Namely, using elacestrant or fulvestrant (eliminating blinding) in place of AI in the control group would have provided a more robust reflection of real-world practice than the continued use of AI in the presence of an ESR1 mutation. Blinding fidelity could still have been maintained if elacestrant was used due to each SERD being administered orally.
I think the investigators chose an appropriate theory to test their hypothesis; however, I also think their theory would benefit from the integration of other components that further explain why patient-reported health status and QoL would be improved by this treatment approach. PFS is only a tenuous surrogate for QoL.5 As such, a more comprehensive theoretical underpinning as to why patients might feel better after this intervention would improve their theory, although I do applaud the inclusion of exploratory QoL aims considering Hwan & Gyawali note that these measures should be an area of increased emphasis in oncology RCTs.
Evaluating Internal Validity6
In the context of threats outlined by Shadish, Cook, and Campbell, we continue our critique of the SERENA-6 study.6 The first potential threat to internal validity in this study is the temporal precedence of when the mutation to ESR1 occurred during the disease course and in relation to the decision to switch treatments. However, the consistent timeline of detection of ESR1 mutation by ctDNA before randomization and follow-up eliminates this threat.
Selection bias could have contributed to the observed effect in the study, as participants who seek clinical trials and receive oncology care at large academic medical centers have been shown to have better outcomes than those who do not.7 However, the multi-center nature of this study helps to mitigate this concern and the size of the effect reported makes this unlikely to be a driving force in the study results.
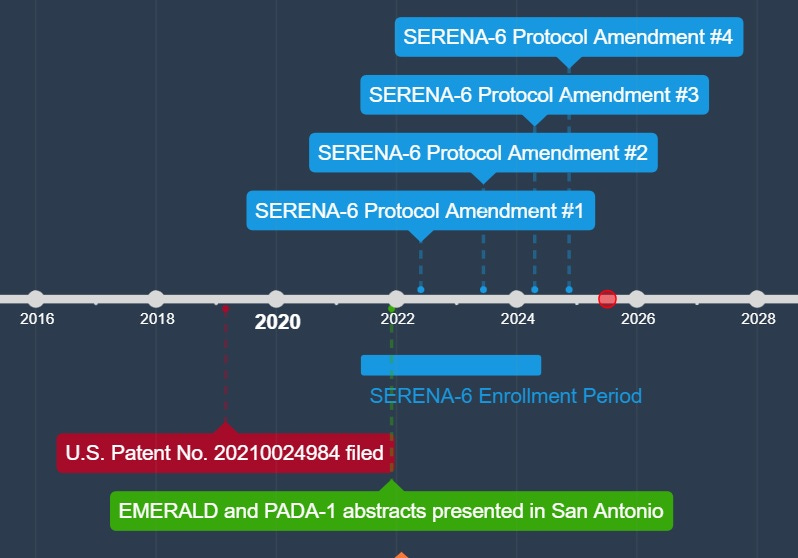
History may have played a role in bringing about the observed effect in the study. Preliminary results of both the PADA-1 and EMERALD studies were reported at the San Antonio Breast Cancer Symposium in December 2021. Enrollment for SERENA-6 began in June 2021 and completed in June 2024, suggesting only a small number of patients had accrued when PADA-1 and EMERALD results were publicly available. Dr. Francois-Clement Bidard is lead author on PADA-1, SERENA-6, EMERALD, and he holds a patent for the laboratory method used to detect the ESR1 mutation, suggesting that the investigator groups on these trials were guided by a sufficient understanding of the literature, treatment guidelines, and underlying biology.1,2,4,9 The first protocol amendment to SERENA-6 was in May 2022, and further protocol amendments were published in March 2023, April 2024 and November 2024. None of the amendments addressed the use of AI in the control group.
Attrition is unlikely to have played a large role in the results of the study after review of the Kaplan-Meier survival curve in Figure 1A. The rates of dropout were consistent with faster disease progression in the control group and there were few instances of censoring which were not due to pre-specified events, supporting the fidelity of blinding.
Instrumentation could have been a factor contributing to the outcomes of the study, but the use of a standardized assay for ctDNA detection as well as an independent central review of clinical progression allows this threat to be effectively mitigated.
Evaluating Statistical Validity6
The exact timing of ctDNA analysis varied in a stated 2-3 month time span. It would have added to the strength of the study if all participants were receiving testing at more precise intervals, but the noise from the timing of these blood draws is probably more reflective or real-world clinical practice and acceptable. Protocol adherence was not explicitly reported in the supplementary appendix or protocol revisions other than a statement in the manuscript espousing that the authors affirm that protocol adherence was optimal. This limits our ability to meaningfully critique this with pill counts or other means of adherence data. There was possibly some variance in adherence as well as data reporting considering the multi-center design, although this was at least partially mitigated by the inclusion of an independent central review.
Evaluating Outcomes and Analyses
I was initially concerned about bias in investigator assessment of disease progression on radiographic scans because measuring the size of a tumor is notoriously prone to measurement error. Investigators may have felt some influence when scoring patients at the margins. However, the blinded independent central review determined an effect similar to the investigators which alleviated my concern (HR = 0.44, 95% CI: 0.31 - 0.60 vs HR = 0.43, 95% CI: 0.29 - 0.63).
I believe that the results of the SERENA-6 trial are promising, but they are marred by a substandard control arm. Although investigators might argue that ctDNA surveillance of emerging mutations was an investigational intervention, the PADA-1 trial showed a strong statistical result suggesting the method’s clinical utility. If we accept the biological premise from PADA-1, I would argue that the equipoise supporting SERENA-6 trial’s stated research question had eroded shortly after enrollment began. This still allowed ample opportunities for protocol amendments to address the deficiency of the control group, especially in the context of EMERALD in which some of the SERENA-6 investigators share authorship. In four protocol amendments, this was not done. Greater care to amend the study design should have been taken to protect participants from a treatment with sufficient evidence of being ineffective.
I would argue that there is equipoise and clinical relevance in asking whether this ctDNA-informed treatment switching strategy optimizes patient outcomes when treating patients with camizestrant, elacestrant, or fulvestrant and the trial groups could have been designed to directly test these SERDs in combination with CDK4/6i after randomization.
Evaluating External Validity6
The inclusion criteria selected for patients that had already received 6 months of treatment without radiologic progression. This chooses for patients that have relatively indolent disease. The KM curve in Figure 1A captures the tail end of the participants with more aggressive tumor biology as demonstrated by a relatively large number of censoring events in the first 3 months of the study in both groups before the treatment effects begin to level out the curves and we see separation. In terms of demographics, the authors note that Black participants were underrepresented in the study, which further limits generalizability of the study findings in addition to patients with more indolent biology.
As mentioned, another variation of treatment (such as elacestrant or fulvestrant) could have resulted in different outcomes, although the pooling of all CDK4/6i is desirable and representative of real clinical practice.
In treatment settings with fewer resources, the implementation of this ctDNA monitoring strategy along with the use of oral SERDs (both of which are more costly than fulvestrant), it is unlikely that real-world patients will have true access to the robust outcomes in PFS demonstrated in SERENA-6. Further efforts to increase access should be explored.
Finally, since our understanding of tumor biology and treatment resistance mechanisms are still evolving, it remains possible that additional resistance mechanisms will become known in time, which will continue to inform our understanding and drive future therapy development.
Evaluation of Rigor, Reporting, and Execution
The study was implemented well considering its massive scope. While specifics were not explicit, the protocol did specify a 75mg dosage of camizestrant and continuation of CDK4/6i. There were no protocol amendments that would have suggested the directors of the study encountered issues with fidelity. Patients were consented and the trial mentions compliance with the Declaration of Helsinki, conduction in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation, international ethical guidelines of the Council for International Organizations of Medical Sciences, and local regulations. To improve study execution, I would suggest that researchers could have taken measures to collect pill counts from participants or prompt patients to keep diaries as a fidelity measure to ensure adherence to the intervention.
Summary and Final Recommendations
The discussion section was congruent and appropriate in context of the research findings. The investigators stated simply that a statistically significant PFS benefit was observed using their treatment strategy, however given their involvement in other trials of SERDs like EMERALD, it was interesting to note that their future directions included only switching studies of CDK4/6i to thwart possible resistance mechanisms to that drug class rather than calling for a more direct comparison of outcomes with elacestrant or fulvestrant.
As this was a phase III RCT, future studies will hopefully expand upon the external validity. While no direct comparison clinical trials of elacestrant and camizestrant are registered at the time of this writing, this would be the next logical step in testing real-world effectiveness. Threats to internal, external, and statistical validity are relatively well balanced. My primary point of contention with the study is its failure to acknowledge other prominent findings in the field that temporally had results which matured around the time this study was enrolling and were authored by some members of this group of investigators. With this in mind, the study as written does further build upon the existing data of PADA-1 that ctDNA monitoring is a viable surveillance strategy to direct treatment decisions and that early detection is better in terms of survival outcomes, but SERENA-6 does not address salient questions that clinicians likely have regarding which SERD to choose in combination with the ctDNA surveillance approach, and this will ultimately be a disappointment to patient advocacy groups.
In summary, three specific changes to improve the study as reported are:
1. Methodologically, remove the placebo from the experimental and control groups to directly test oral SERDs camizestrant vs elacestrant vs fulvestrant (in a third experimental arm). This removes blinding but answers an important clinical question with objective survival endpoints.
2. Targeted inclusion of Black patients to ensure a more representative clinical sample. Racial disparities in breast cancer survivorship are well documented in the literature.10,11 That n=47 (1.6%) of N=2885 patients on the study self-identified as Black in a study designed to be performed in 264 sites across 23 countries is a significant underrepresentation. This could have been accomplished most directly by choosing sites for screening based on the demographics of the patients served, improving catchment.
3. A concerted effort to collect and interpret adherence data. Smart pill bottles could have been implemented to track and collect data regarding whether patients were self-administering the oral SERD appropriately, which would have contextualized findings with relevant adherence data.
References
1 Bidard, F.-C., Mayer, E. L., Park, Y. H., Janni, W., Ma, C., Cristofanilli, M., Bianchini, G., Kalinsky, K., Iwata, H., Chia, S., Fasching, P. A., Brufsky, A., Nowecki, Z., Pascual, J., Moreau, L., Chen, S.-C., Karadurmus, N., Gal-Yam, E. N., Jung, K. H., … Turner, N. C. (2025). First-line camizestrant for emerging ESR1-mutated advanced breast cancer. New England Journal of Medicine. https://doi.org/10.1056/NEJMoa2502929
2 Bidard, F.-C., Hardy-Bessard, A.-C., Dalenc, F., Bachelot, T., Pierga, J.-Y., Rouge, T. de la M., Sabatier, R., Dubot, C., Frenel, J.-S., Ferrero, J. M., Ladoire, S., Levy, C., Mouret-Reynier, M.-A., Lortholary, A., Grenier, J., Chakiba, C., Stefani, L., Plaza, J. E., Clatot, F., … Gramont, A. D. (2022). Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. The Lancet Oncology, 23(11), 1367–1377. https://doi.org/10.1016/S1470-2045(22)00555-1
3 Oliveira, M., Pominchuk, D., Nowecki, Z., Hamilton, E., Kulyaba, Y., Andabekov, T., et al. (2024). Camizestrant, a next-generation oral SERD, versus fulvestrant in post menopausal women with estrogen receptor-positive, HER2-negative advanced breast cancer (SERENA-2): A multi-dose, open-label, randomized phase 2 trial. Lancet Oncology, 25(11), 1424-39. DOI: 10.1016/S1470-2045(24)00387-5
4 Bidard, F.-C., Kaklamani, V. G., Neven, P., Streich, G., Montero, A. J., Forget, F., Mouret-Reynier, M.-A., Sohn, J. H., Taylor, D., Harnden, K. K., Khong, H., Kocsis, J., Dalenc, F., Dillon, P. M., Babu, S., Waters, S., Deleu, I., García Sáenz, J. A., Bria, E., … Bardia, A. (2022). Elacestrant versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: Results from the randomized phase III EMERALD trial. Journal of Clinical Oncology, 40(28), 3246–3256. https://doi.org/10.1200/JCO.22.00338
5 Hwang, T. J., & Gyawali, B. (2019). Association between progression-free survival and patients’ quality of life in cancer clinical trials. International Journal of Cancer, 144(7), 1746–1751. https://doi.org/10.1002/ijc.31957
6 Shadish, W. R., Cook, T. D., & Campbell, D. T. (2002). Experimental and quasi-experimental designs for generalized causal inference (2nd ed.). Houghton Mifflin.
7 Du Bois, A., Rochon, J., Lamparter, C., Pfisterer, J. (2005). Pattern of care and impact of participation in clinical studies on the outcome in ovarian cancer. Int J Gynecol Cancer, 15, 183-191.
8 Bidard FC, Hardy-Bessard AC, Bachelot T, et al. (2021). Fulvestrant-palbociclib vs continuing AI-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2– metastatic breast cancer patients: Results of PADA-1, a UCBG-GINECO randomized phase 3 trial. 2021 San Antonio Breast Cancer Symposium. Abstract GS3-05. Presented December 9, 2021.
9 Stern, M. H., Jeannot, E., Proudhon, C., Pierga, J. Y., Bidard, F. C. (2021). Method for identifying one or more mutations in a hotspot mutation sequence (U.S. Patent No. 20,210,024,984). U.S. Patent and Trademark Office. https://patents.justia.com/inventor/francois-clement
10 Rosenzweig, M. Q., & Mazanec, S. R. (2023). Racial differences in breast cancer therapeutic toxicity: Implications for practice. Cancer Epidemiology, Biomarkers & Prevention, 32(2), 157–158. https://doi.org/10.1158/1055-9965.EPI-22-1111
11 Puthanmadhom Narayanan, S., Ren, D., Oesterreich, S., Lee, A. V., Rosenzweig, M. Q., & Brufsky, A. M. (2023). Effects of socioeconomic status and race on survival and treatment in metastatic breast cancer. Npj Breast Cancer, 9(1), 90. https://doi.org/10.1038/s41523-023-00595-2